Abstract
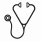
Background
Clonal chromosomal abnormalities in Philadelphia chromosome-negative metaphases (CCA/Ph-) emerge as patients (pts) with chronic phase chronic myeloid leukemia (CP-CML) are treated with tyrosine kinase inhibitors (TKI). The clinical significance of CCA/Ph- remains unclear specifically among pts treated with second generation TKI. Very little is known about the molecular abnormalities associated with them.
Methods
We conducted a retrospective analysis of pts enrolled on prospective, single-institution frontline trials for CML-CP. Of the 598 pts enrolled, 279 pts (46%) were treated with imatinib, 126 (21%) with nilotinib, 143 (23%) with dasatinib and 50 (8%) with ponatinib. CCA/Ph- were defined as abnormalities present in ≥2/20 Philadelphia-negative (Ph-) metaphases or if present in one metaphase in ≥2 cytogenetic assessments. The clinical outcomes of pts with CCA/Ph- were compared to a control group without additional chromosomal abnormalities (ACAs). We performed targeted deep sequencing (median depth 490x) on a pilot study from 10 pts with various CCA/Ph- (panel of 295 genes recurrently mutated in hematological malignancies). Single nucleotide variants (SNV) and small insertions and deletions (indels) were called against virtual normal sequence developed in-house. An automatic pipeline was used to filter and annotate raw variants, and then select non-polymorphism, protein-coding changing mutation, thus identifying high confidence driver mutations.
Results
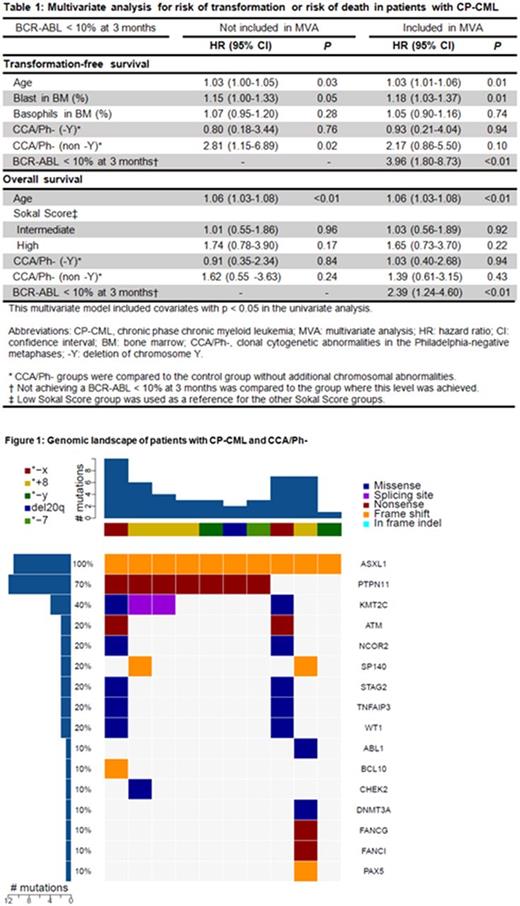
CCA/Ph- occurred in 58 pts (10%) and the most common were - Y in 25 (43%) and trisomy 8 in 7 pts (12%). Most CCA/Ph- appeared within the first year of the start of the TKI (median 6 months, range 3-78 months). CCA/Ph- were transient and were detected in 2 or less time points in 36/58 cases (62%). The median number of Ph-negative metaphases containing CCA/Ph- per analysis was 5/20 (range 1-20). We further categorized CCA/Ph- into those where - Y was the only clonal abnormality, and all others. Pts who developed CCA/Ph- were significantly older than those without ACAs (median age 59 vs 47; P<0.001). Specifically, pts with -Y CCA/Ph- were the oldest (median age 64; P<0.001), followed by those with non-Y CCA/Ph- (median age 52; P=0.003) when compared to those without ACAs (median age 47). Four pts with ACAs in Ph+ metaphases who subsequently developed CCA/Ph- were excluded from response and survival analyses. Response to TKI therapy was similar for pts with CCA/Ph- and those without ACAs. With a median follow-up for survivors of 7.6 years (range 0.2-14.7 years), we found that pts with non -Y CCA/Ph- had worse failure-free survival (FFS), event-free survival (EFS), transformation-free survival (TFS) and overall survival (OS) compared to those without ACAs with the following 5-year rates: FFS (52% vs 70%, P = 0.02), EFS (68% vs 86%, P = 0.02), TFS (76% vs 94%, P < 0.01) and OS (79% vs 94%, P = 0.03). In a multivariate analysis, non -Y CCA/Ph- increased the risk of transformation or death when baseline characteristics were considered with a hazard ratio (HR) of 2.81 (95% CI 1.15-6.89, P = 0.02). However, this prognostic impact was not statistically significant when achieving BCR-ABL < 10% at 3 months was included in the analysis (Table 1).
A total of 46 high-confidence driver mutations in 18 genes in 10/10 (100%) pts with CCA/Ph- were identified (Figure 1). Median number of driver mutations was 2.5 (range 1-9). ASXL1 frameshift mutation was detected in all examined pts (10/10 pts) mostly at low variant allelic frequency (VAF) with a median VAF of 1.6% (range 0.7% - 4.2%). Mutations were also commonly detected in PTPN11 (7/10 pts). Mutations in PTPN11 have been reported in about 4% of pts with acute myeloid leukemia (Papaemmanuil et al. NEJM 2016). Mutations in DNMT3A (R635Q) and ABL (Y253H, associated with resistance to imatinib) were observed in the same case in addition to ASXL1. In this cohort, the observed mutation rate is higher than what is expected in CP-CML when comparing to published literature (Kim et al. Blood 2017). Sequencing of a control cohort from pts with deep molecular response without ACAs is ongoing.
Conclusion
In conclusion, non -Y CCA/Ph- are associated with decreased survival when emerging in pts with chronic phase CML across various TKIs. Preliminary results from deep sequencing indicate a higher than expected rate of mutations in CP-CML pts with CCA/Ph- compared to those without ACAs, mostly detected at low VAF.
Kantarjian: Pfizer: Research Funding; Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Novartis: Research Funding; Amgen: Research Funding; Delta-Fly Pharma: Research Funding. Takahashi: Symbio Pharmaceuticals: Consultancy. Jabbour: Bristol-Myers Squibb: Consultancy. Cortes: ARIAD: Consultancy, Research Funding; Sun Pharma: Research Funding; BMS: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Teva: Research Funding; ImmunoGen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract